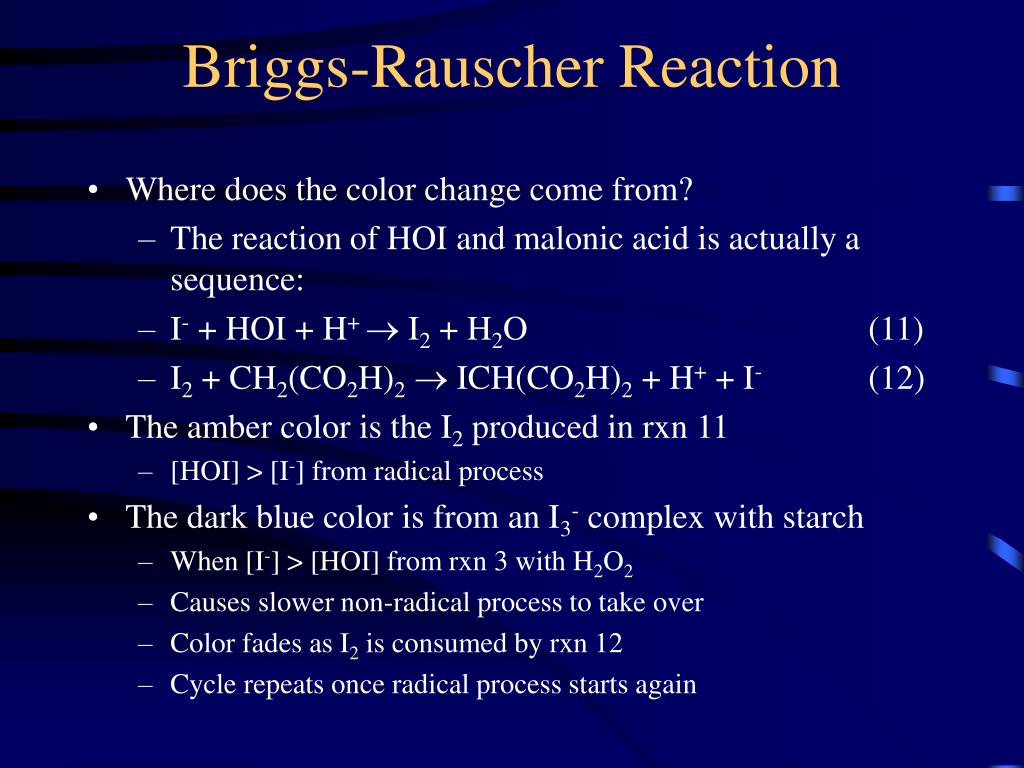
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
Reaksi Briggs-Rauscher, juga dikenal sebagai 'jam berosilasi', adalah salah satu demonstrasi paling umum dari reaksi osilator kimia. Reaksi dimulai ketika tiga larutan tanpa warna dicampur bersama. Warna campuran yang dihasilkan akan berosilasi antara jernih, kuning, dan biru tua selama sekitar 3-5 menit..

Briggs Rauscher Oscillating Chemical Reaction YouTube
Skripsi ini bertujuan untuk mempelajari reaksi Briggs-Rauscher (BR) yang merupakan reaksi berosilasi. Model reaksi BR oleh De Kepper dan Epstein yang digunakan telah disederhanakan dari 15 variabel konsentrasi dan 10 tahap reaksi sehingga hanya terdiri dari 10 variabel konsentrasi. Konstruksi model menggunakan hukum aksi massa yang memberikan sebuah sistem dari 10 persamaaan diferensial.

Briggsrauscher Oscillating Reaction Photograph by Science Photo Library Pixels
ABSTRACT: The Briggs Rauscher reaction is a popular − demonstration to illustrate chemical oscillations in laboratories, classrooms, and public seminars because of its simplicity and visual appeal. Here, we adapt the Briggs Rauscher reaction to present − reaction diffusion convection patterns in the undergraduate − general or physical.

A BriggsRauscher Reaction YouTube
Reaksi Briggs-Rauscher adalah salah satu contoh reaksi osilasi. Reaksi ini cocok digunakan untuk keperluan peragaan karena perubahan warnanya yang mencolok: solusi tak berwarna yang telah disiapkan beberapa saat sebelumnya secara perlahan berubah menjadi warna batu ambar, dan kemudian secara tiba-tiba menjadi biru tua. Warna ini secara perlahan hilang dan lalu proses ini berulang sekitar.

X870 BriggsRauscher Reaction Oscillating Clock Lecture Demonstration Manual General
The Briggs-Rauscher oscillating reaction is one of the very few oscillating reactions that we know of. Throughout the reaction, the concentration of iodide (.

How To Do BriggsRauscher Reaction At Home Diy YouTube
The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction begins when three colorless solutions are mixed together. The color of the resulting mixture will oscillate between clear, amber, and deep blue for about 3-5 minutes.

"Iodine clock" experiment (the BriggsRauscher reaction) MEL Chemistry
The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, then suddenly changes to a very dark blue..

Chart of regimes of oscillations of the BriggsRauscher reaction. The... Download Scientific
The Briggs-Rauscher reaction involving iodate, hydrogen peroxide, malonic acid and Mn(II) in acid solution exhibits bistability and oscillation in a CSTR as well as batch oscillation. We have developed a mechanism for this reaction which reproduces these and other dynamic features. Astonishingly, our model is qualitatively identical to, though.

BriggsRauscher Reaction MIT Chemistry Behind the Magic YouTube
MIT Chemistry Behind the MagicView the complete course: http://ocw.mit.edu/behindthemagicInstructor: John Dolhuh, Jessica HarropLicense: Creative Commons BY-.

BriggsRauscher Berubah Warna Berubah Reaksi Matematika Ilmu Teknologi 2024
Briggs Rauscher Reaction. This is the Briggs Rauscher reaction, the coolest chemistry demonstration on the planet. This is an oscillating reaction, which means the reactants will form products, which will reform the reactants several times, until one of the reactants is extinguished and the reaction stops. The blue color is an iodine-starch.

The BriggsRauscher Reaction An Oscillating Chemical Reaction
Reaksi Briggs-Rauscher, juga dikenal sebagai 'jam berosilasi', adalah salah satu demonstrasi paling umum dari reaksi osilator kimia. Reaksi dimulai ketika tiga larutan tidak berwarna dicampur bersama-sama. Warna campuran yang dihasilkan akan terombang -ambing antara bening, kuning, dan biru tua selama sekitar 3-5 menit.

BriggsRauscher Reaktion Flaskman
A Potentiometric Study of the Briggs-Rauscher Oscillatory Reaction in a Solution of Ionic Surfactants and Tetrabutylammonium Hydrogensulphate. ChemistrySelect 2018 , 3 (39) , 10951-10958.

BriggsRauscher Oscillating Color Change Villanova College Chemistry Blog
Oscillating magic: the Briggs-Rauscher oscillating reaction. By Declan Fleming 2019-02-25T11:39:00+00:00. No comments. Make your students' jaws drop with this colour change reaction. Arthur C Clarke's famous third law: 'any sufficiently advanced technology is indistinguishable from magic' can also be applied to chemistry. There's.

Reacción de BriggsRauscher (Un reloj químico) YouTube
Quenching Analysis of the Permanganate−Hydroxylamine Oscillator. The Journal of Physical Chemistry A 1997, 101 (7) , 1317-1323. DOI: 10.1021/jp962559k. V. Vukojević,, P. Graae Sørensen, and, F. Hynne. Predictive Value of a Model of the Briggs−Rauscher Reaction Fitted to Quenching Experiments. The Journal of Physical Chemistry 1996, 100.

Briggs Rauscher Reaction ChemTalk
Briggs-Rauscher reaction. The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking color changes: the freshly prepared colorless solution slowly turns an amber color, suddenly changing to a very dark blue.
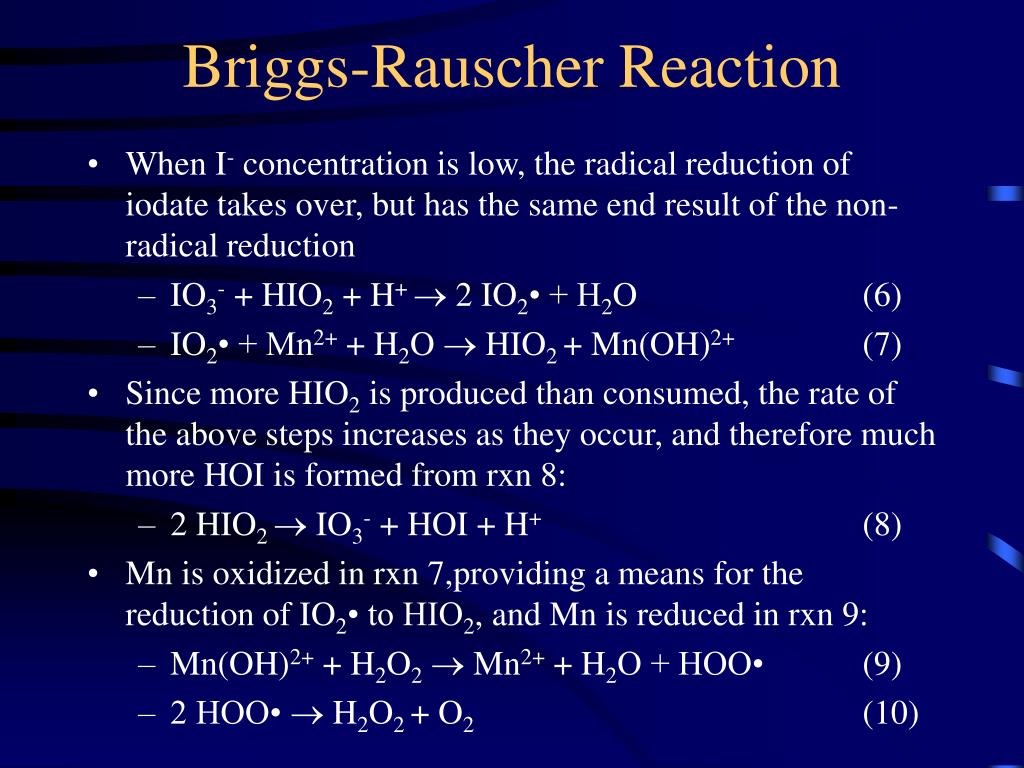
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
The Briggs-Rauscher reaction is an oscillating clock reaction that cycles through colors. The Briggs-Rauscher reaction is an oscillating clock chemical reaction that cycles from clear, amber, to deep blue several times before finally ending as a blue-black mixture. It is one of the most popular chemistry demonstrations because it's easy and reliable.