PPT Energetics PowerPoint Presentation, free download ID836262
This chemistry video tutorial explains the concept of hess' law and how to use it to find the enthalpy change of a reaction by finding the heat of summation.

Chapter 09 20 Hess's Law of Heat Summation (graphically) YouTube
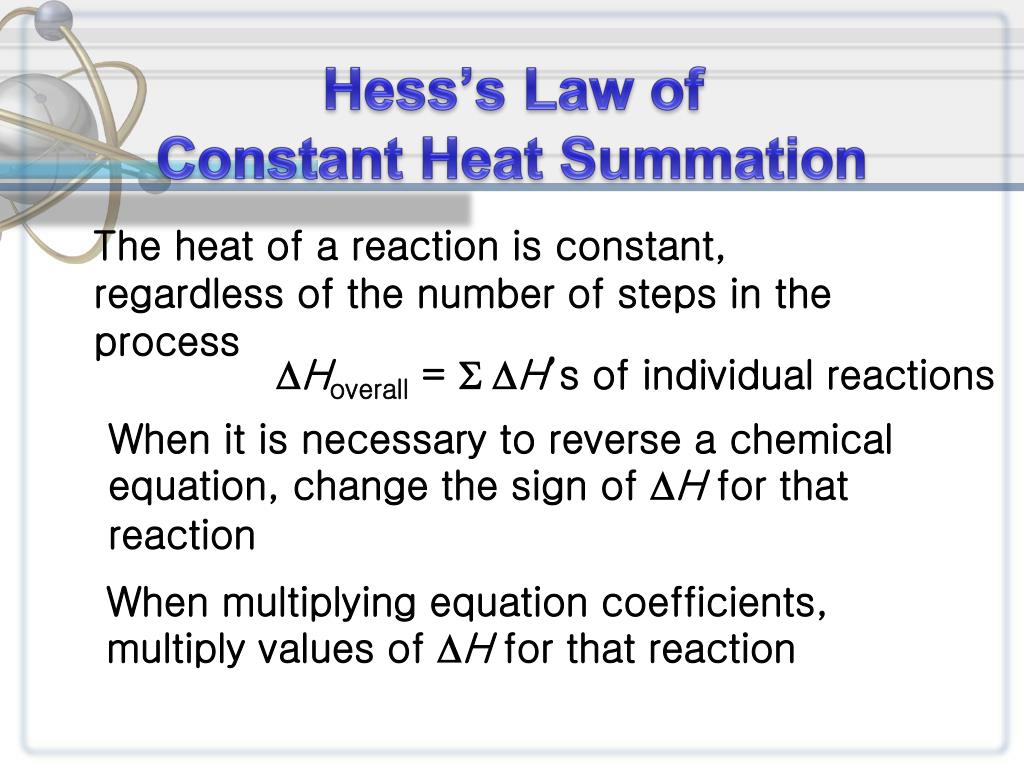
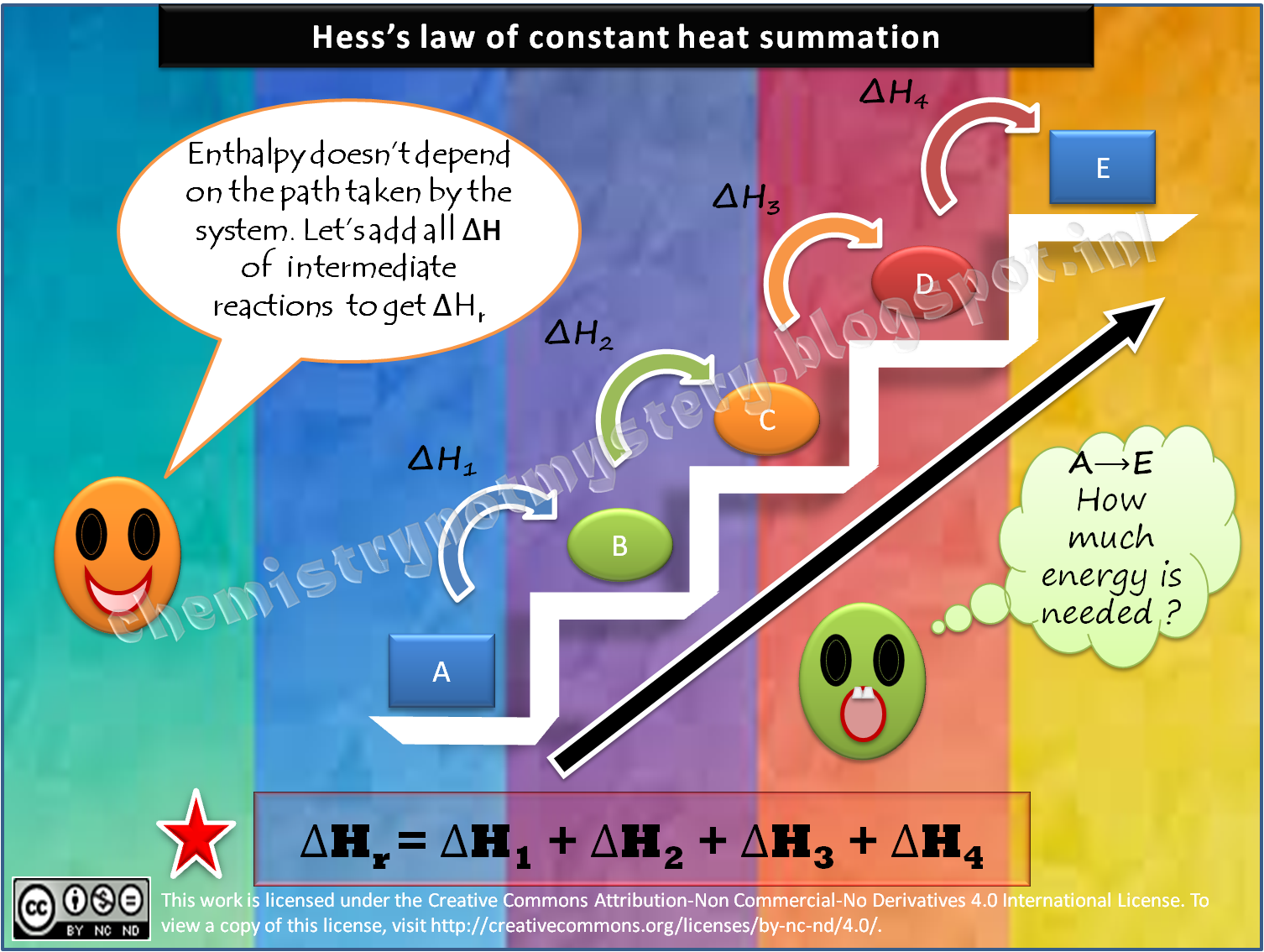
Hess's Law of Constant Heat Summation states that r, the total enthalpy change for the reaction is the sum of all changes and does not depend whether it takes place in single or multiple steps. This law is aan outcome of the fact that enthalpy is a state function. The applications of Hess's law are in . Calculation of enthalpy of formation

Hess's Law of Constant Heat Summation, Chemistry Lecture Sabaq.pk
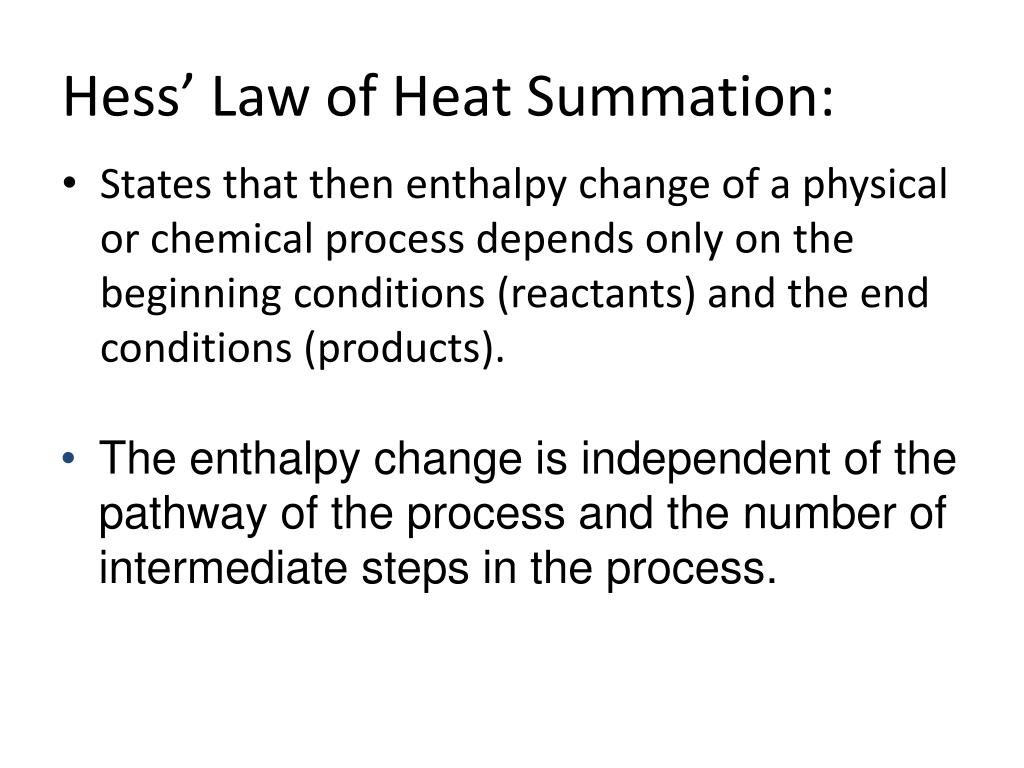
Understanding Hess Law. Hess's Law, otherwise known as the law of constant heat summation, is a fundamental principle in the field of chemistry. This law asserts that the total enthalpy change (ΔHrec) in a chemical reaction remains constant, regardless of the reaction pathway taken, provided the temperature remains constant.

Thermodynamics Class 11/ Part 22/ Hess's law of constant heat summation
The purpose of Hess's law is to measure the enthalpies of neutralization for several acid-base reactions, then use that information and Hess's law to determine the reaction enthalpies for two salts in aqueous solution. Table of Contents Application of Hess's Law Determination of Enthalpy of Formation Calculation of Standard Enthalpies of Reaction
.PNG)
Hess's Law Presentation Chemistry
Applications of Hess's law of constant heat summation. This law can be used to determine the heat of the formation of a substance that cannot be measured experimentally. For example, the formation of benzene can not be prepared by combining the individual atoms such as carbon and hydrogen.
Chemical thermodynamics lesson plan 1 Types of
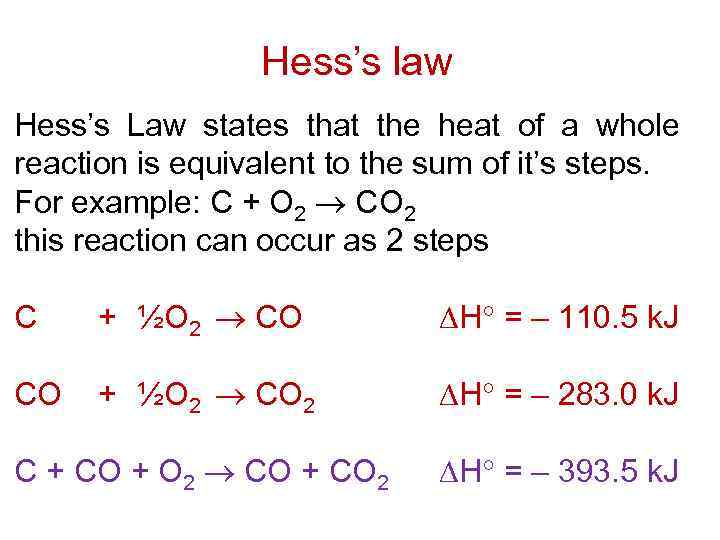
Hess's law of heat summation states that the total enthalpy change during a reaction is the same whether the reaction takes place in one step or in several steps. For example, in the above diagram, ΔH 1 = ΔH 2 + ΔH 3 = ΔH 4 + ΔH 5 +ΔH 6. In Hess's Law calculations, you write equations to make unwanted substances cancel out.

Hess's Law of Constant Heat Summation Thermodynamics NCERT
17: Thermochemistry
Hess Law chemistryatdulwich
Therefore, the definition of Hess law of constant heat summation is: "The enthalpy change in a chemical reaction is the same and independent of the path or steps the reaction takes." Explain Hess's Law with Example Let us take an example to understand the Hess law of constant heat summation. Example 1

Applying Hess's Law of Constant Heat Summation YouTube
The Hess's law can also be stated as the enthalpy change for a chemical reaction is the same regardless of the path by which the reaction occurs. For example, consider following two paths for the preparation of methylene chloride Path I : CH 4(g)+2Cl2(g) → CH 2Cl2(g)+2H Cl(g) ΔH 0 1 = −202.3kJ Path II :
Chem 1045 Lab hesss_law
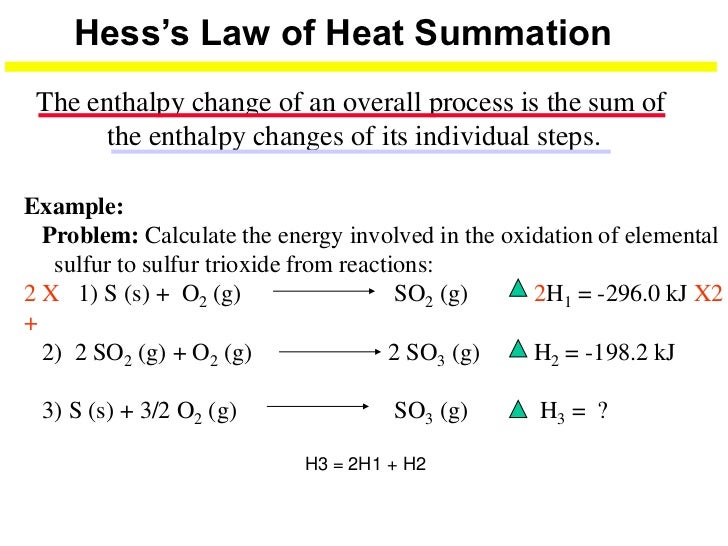
Hess's law says that the heat of a reaction is constant whether it is carried out directly in one step or indirectly in a series of steps. If you reverse a chemical reaction, the sign of ΔH must be changed. If you multiply a chemical reaction by a number, then ΔH must also be multiplied by that number. first arrange the equations so that.

PPT Energetics PowerPoint Presentation ID1204656
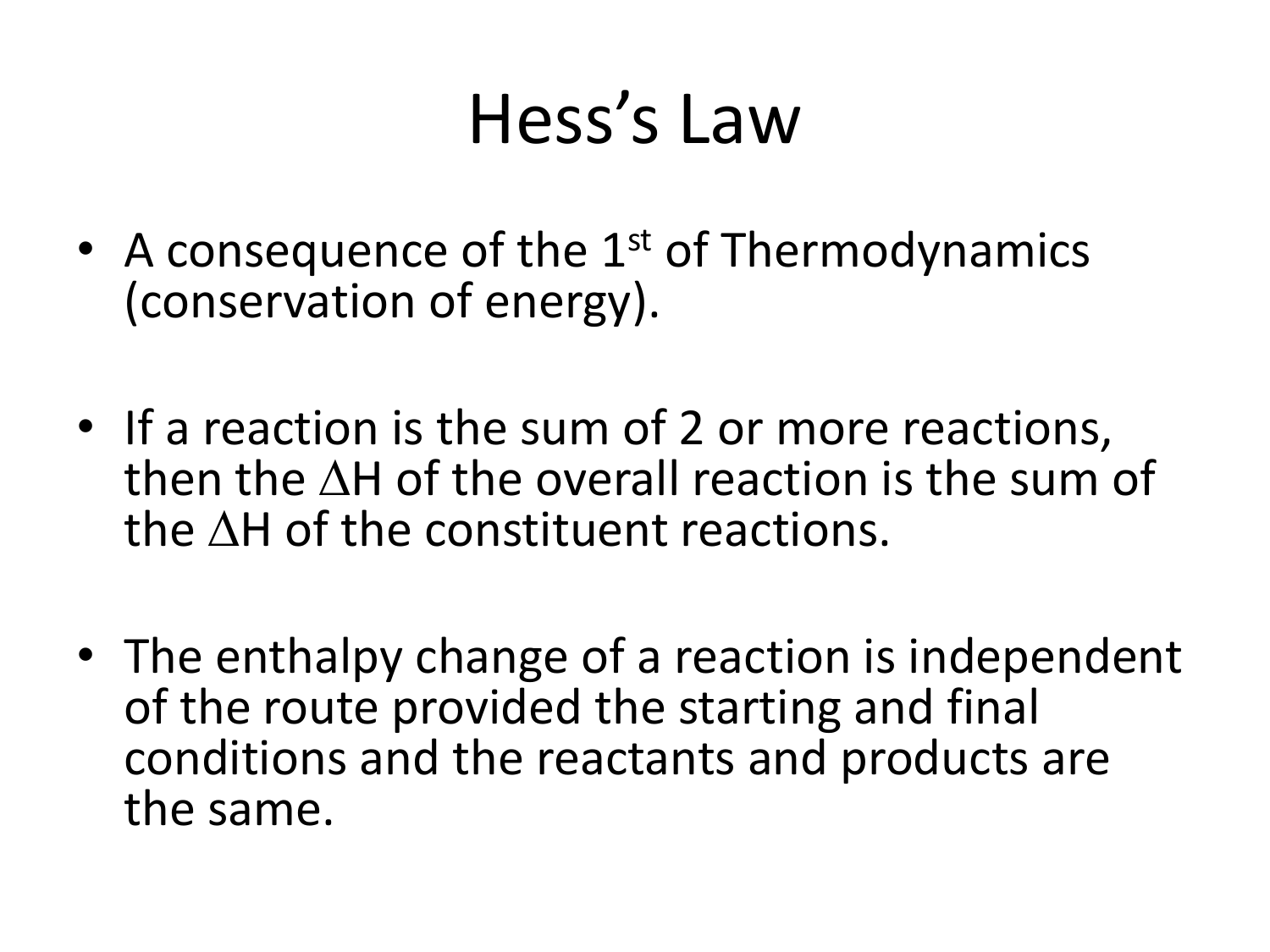
Additionally known as the Hess law of constant heat summation, is one of the key consequences of the first law of thermodynamics. Whether a chemical or physical process is carried out in one step or several steps, the enthalpy change is the same in both cases.

PPT Hess’s Law PowerPoint Presentation, free download ID6193634
This type of calculation usually involves the use of Hess's law, which states: If a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps.

Hess Law of Heat Summation
Hess's law, also called Hess law of constant heat summation, is one of the important outcomes of the first law of thermodynamics. The enthalpy change in a chemical or physical process is similar whether it is carried out in one step or in several steps.
chemistry Hess’s Law of Constant Heat Summation
Hess's law of constant heat summation states that the total enthalpy change in a particular reaction is constant regardless whether it occurs in one step or more. Explanation of Hess's Law According to Hess's law, if A reacts to form the product B, it doesn't matter how many steps involved to get the product, the total enthalpy change will be same.
PPT Hess’ Law of Heat Summation PowerPoint Presentation, free
Hess's law of constant heat summation, also known simply as Hess' law, is a relationship in physical chemistry named after Germain Hess, a Swiss -born Russian chemist and physician who published it in 1840. The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken.

Hess’s Law of Constant Heat Summation Physics & Mathematics Physics
Energy (enthalpy) of a system (molecule) is a state function. So, enthalpy of reactant and product molecules is a constant and does not change with origin and path of formation. The first law of thermodynamics states that the total energy of the substances before and after any (physical or chemical) change should be equal.