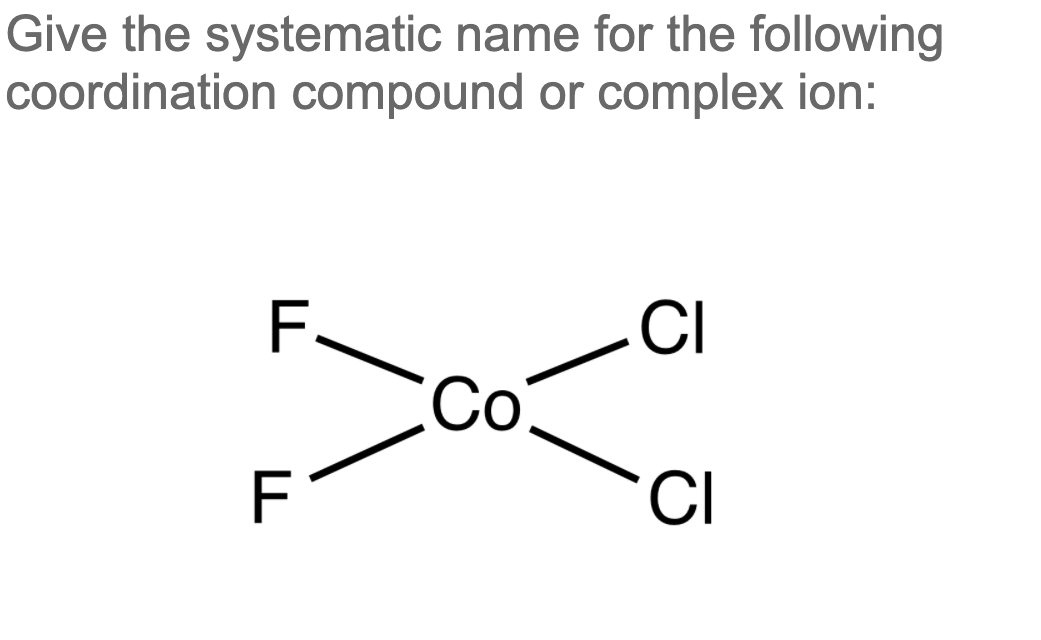
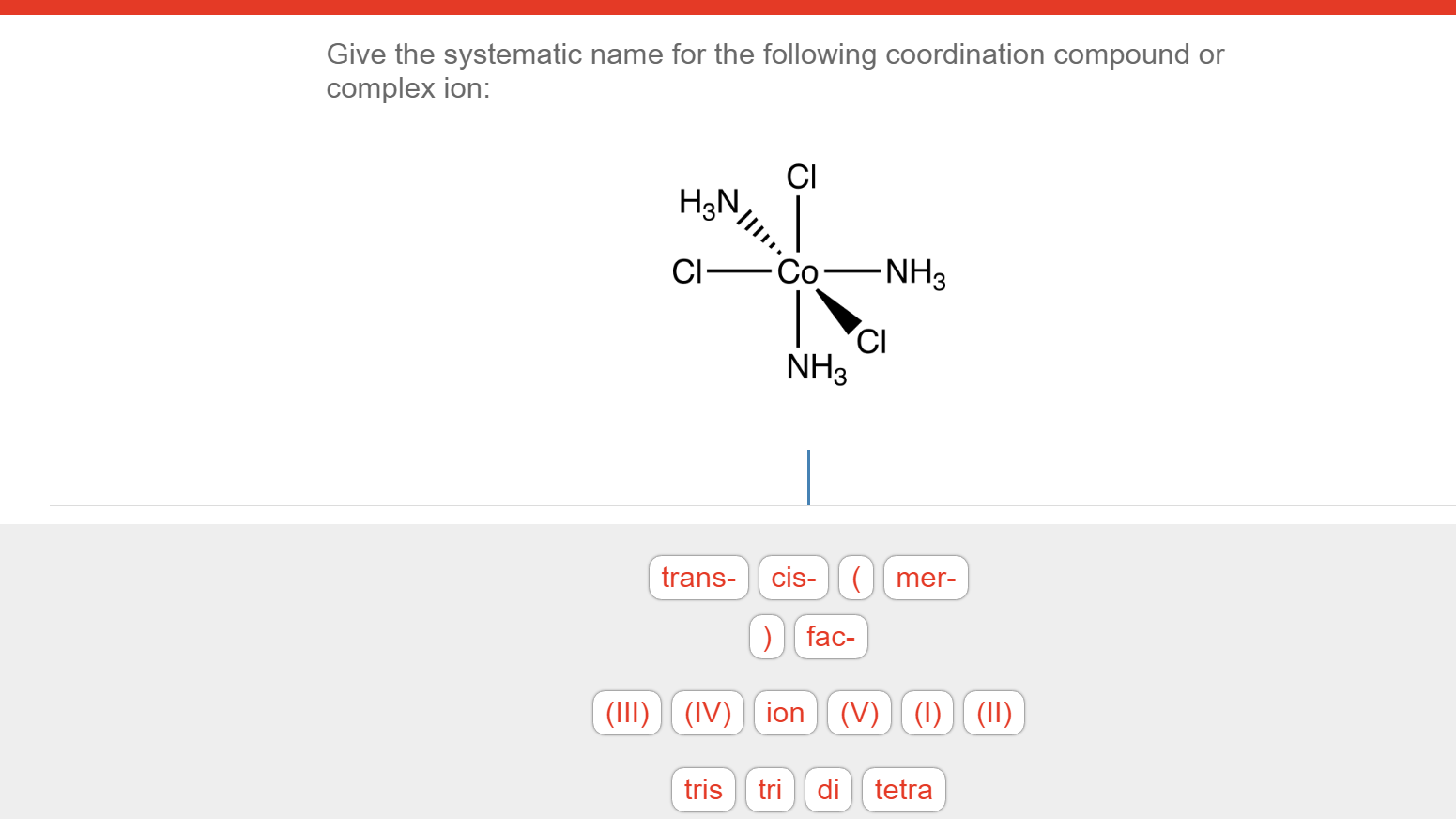
Solved Give the systematic name for the following
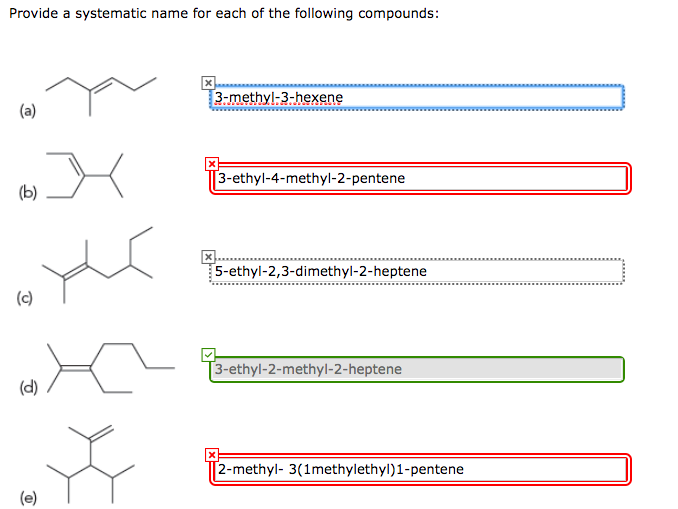
How to get a systematic name from a structure To assign a name to a compound, begin by determining the ' parent chain ', which is the longest straight chain of carbon atoms. On paper, you should be able to put a finger down on one end of the parent chain and trace through all carbons until you get to the end, without needing to lift your finger.
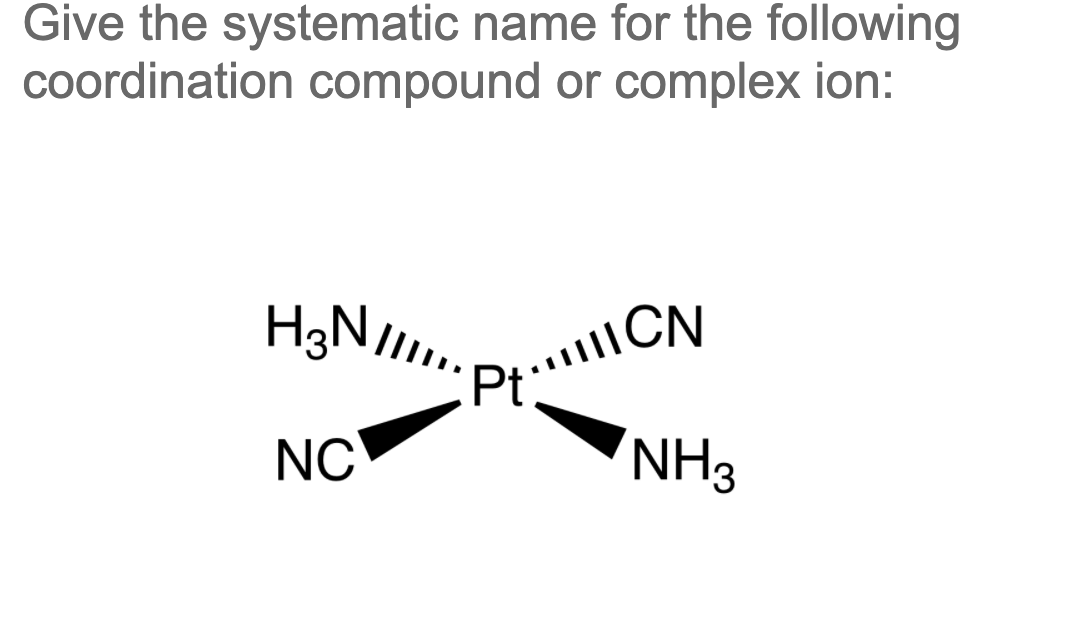
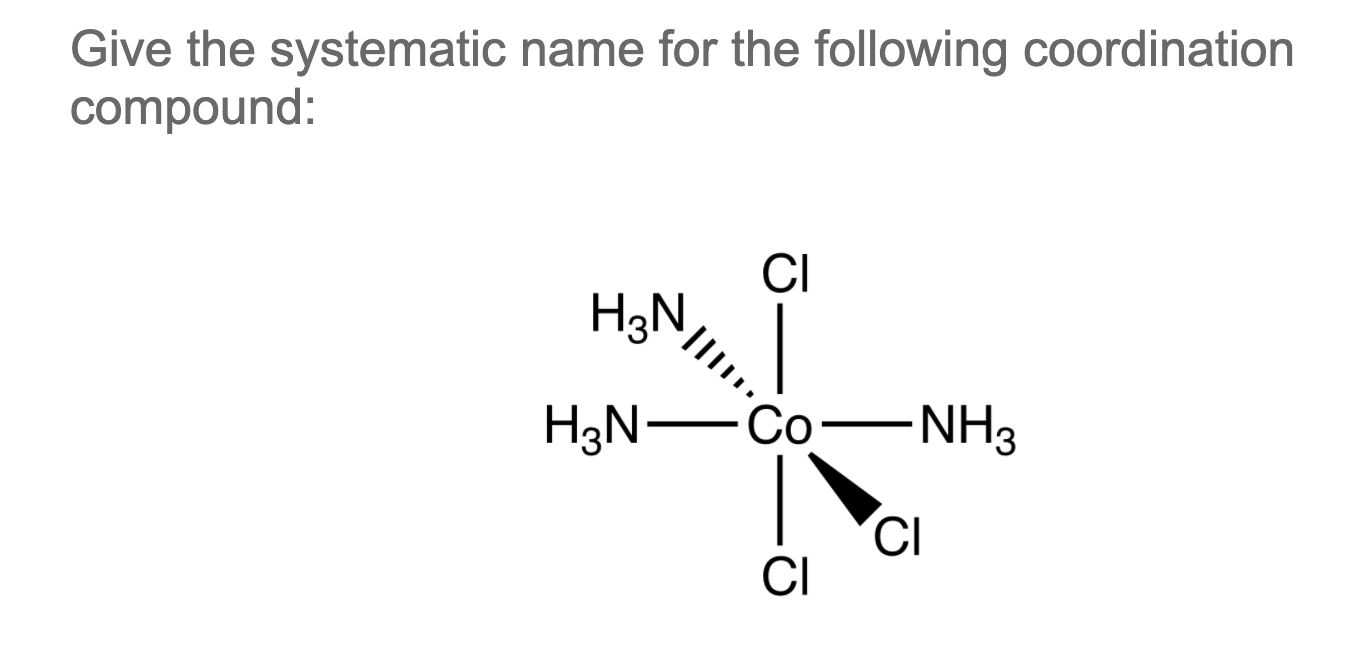
Solved Give the systematic name for the following
For many compounds, the systematic name and the common name are both used frequently, requiring familiarity with both. For example, the systematic name for NO is nitrogen monoxide, but it is much more commonly called nitric oxide. Similarly, N 2 O is usually called nitrous oxide rather than dinitrogen monoxide.
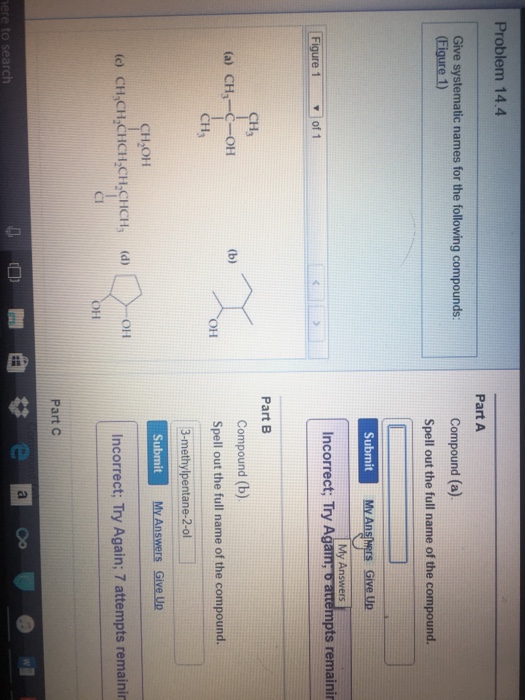
Solved Give systematic names for the following compounds.
The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound's name is 4-methyl-2-pentene.

Solved Give systematic names for the following compounds
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix - ide ). Some examples are given in Table 2.6. Names of Some Ionic Compounds Table 2.6 Compounds Containing Polyatomic Ions
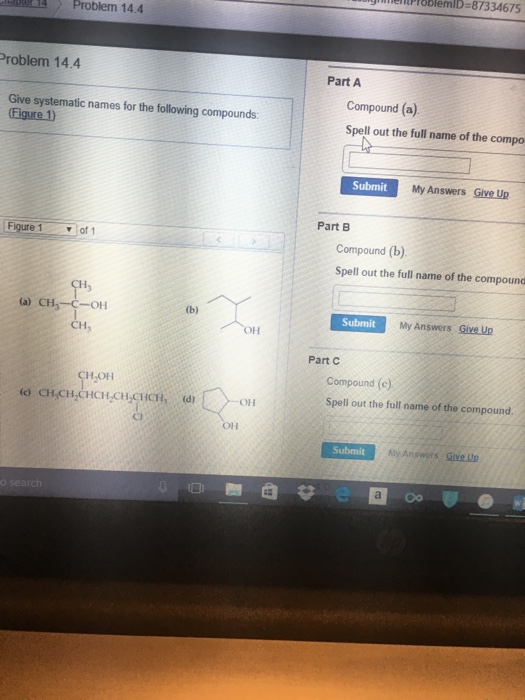
Solved Give systematic names for the following compounds
This nomenclature will be discussed when it is possible for a functional group. 3.2: Overview of the IUPAC Naming Strategy is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The International Union of Pure and Applied Chemistry (IUPAC) names for organic compounds all follow the same set of rules.
Solved Give the systematic name for the following
Give systematic names for the following compounds: (a) NCl3 (b) P4O6 (c) S2F2 Name the following binary compounds of nitrogen and oxygen: (a) NO (b) N2O (c) NO2 (d) N2O4 (e) N2O5 Name the following binary compounds of sulfur and oxygen: (a) SO (b) S2O2 (c) S5O (d) S7O2 (e) SO3
SOLVEDGive the systematic name for each of the following compounds
This is a set of practice problems on naming organic compounds. The examples cover the nomenclature of alkanes, bicyclic compounds, alkenes, alkynes, alcohols, alkyl halides, aromatic compounds, aldehydes and ketones, amines, ethers, and carboxylic acid derivatives such as nitriles, esters and amides.

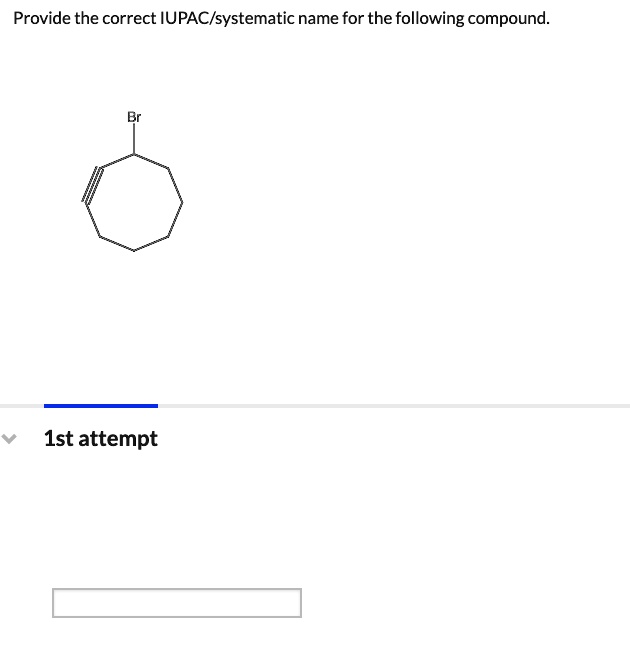
Provide The Correct Iupac/Systematic Name For The Following Compound.
Question: Give systematic names for the following compounds. (Figure 1) Compound (a). Spell out the full name of the compound. Compound (b). Spell out the full name of the compound. Compound (c). Spell out the full name of the compound. Show transcribed image text Here's the best way to solve it. Expert-verified 100% (1 rating) Share Share
Solved Give the systematic name for the following
Table 6.1 lists the common (trivial) names of some molecular compounds. Several ionic compounds are listed in Table 6.2, with both their common and systematic names. TABLE 6.2 Names and formulas of some common ionic compounds. Common name. Systematic name. Formula. bleach. sodium hypochlorite. NaOCl.
SOLVED Provide the correct IUPAC/systematic name for the following compound. 1st attempt
There are 2 steps to solve this one. Expert-verified 100% (2 ratings) Step 1 To name a coordination complex, follow these steps: Identify the cation and write its name first. Thi. View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text:
Provide a Systematic Name of the Following Compound Below MarenhasColeman
Learn Test Match Q-Chat Created by carrots92 Terms in this set (6) Classify each of the following compounds as ionic or molecular. HCN CCl4 NI3 PtO2 Cr2O3 Ionic: PtO2 Cr2O3 Molecular: HCN CCl4 NI3 Give the systematic name for the compound Mg (NO3)2.

SOLVEDGive the systematic name for each of the following compounds
Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Second, name the other nonmetal by its element name, but shorten its name and add an -ide.
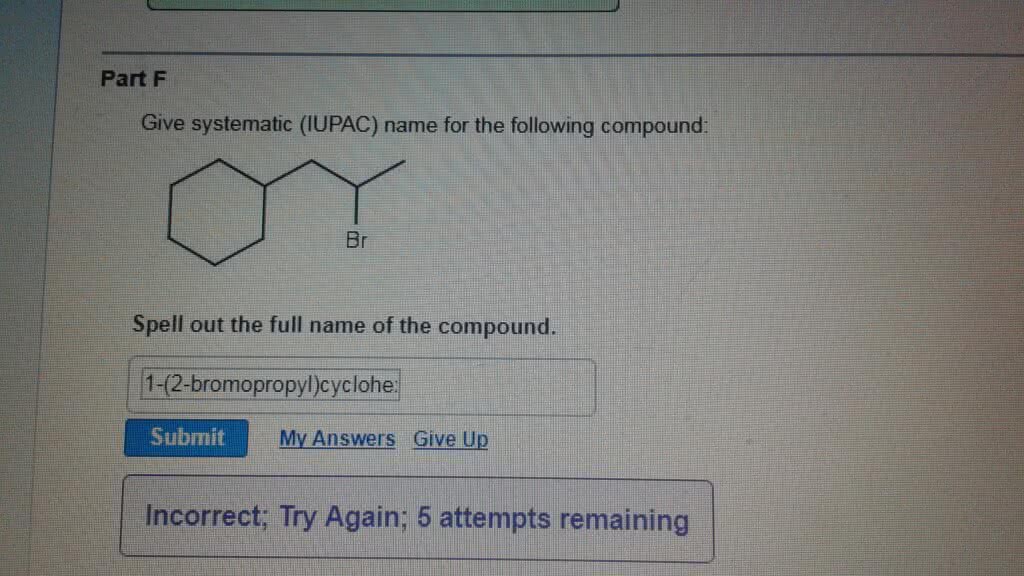
OneClass Part F Give systematic (IUPAC) name for the following compound Br Spell out the full
Example 10.3.1. Write the systematic name (and the common name if applicable) for each ionic compound. LiCl; MgSO 4 (NH 4) 3 PO 4; Cu 2 O; Given: empirical formula Asked for: name Strategy: A If only one charge is possible for the cation, give its name, consulting Table 10.1.2 or Table 10.2.1 if necessary. If the cation can have more than one charge (Table 10.3.1), specify the charge using.

Solved Give systematic names for the following compounds
What is the systematic name of the following compound? Al 4 C 3 Choose 1 answer: Silver carbonate A Silver carbonate Silver carbide B Silver carbide Aluminum carbide C Aluminum carbide Aluminum carbonate D Aluminum carbonate Show Periodic Table Stuck? Use a hint. Report a problem Do 4 problems
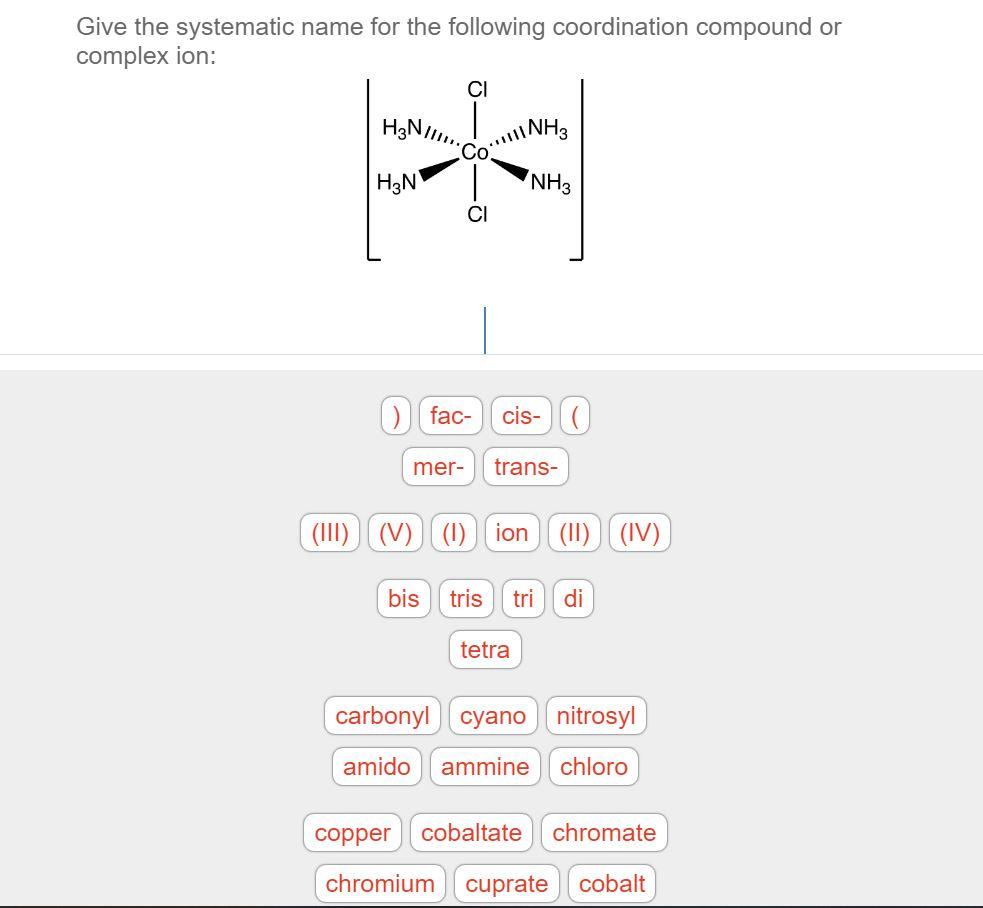
Solved Give the systematic name for the following
In this task, we need to give a systematic name to three different compounds. All three elements (nitrogen, oxygen and sulfur) are nonmetals, so we need to list the rules for naming molecular covalent compounds: The first element in the formula is named using the full element name. The second element is named as an anion with the suffix -ide.
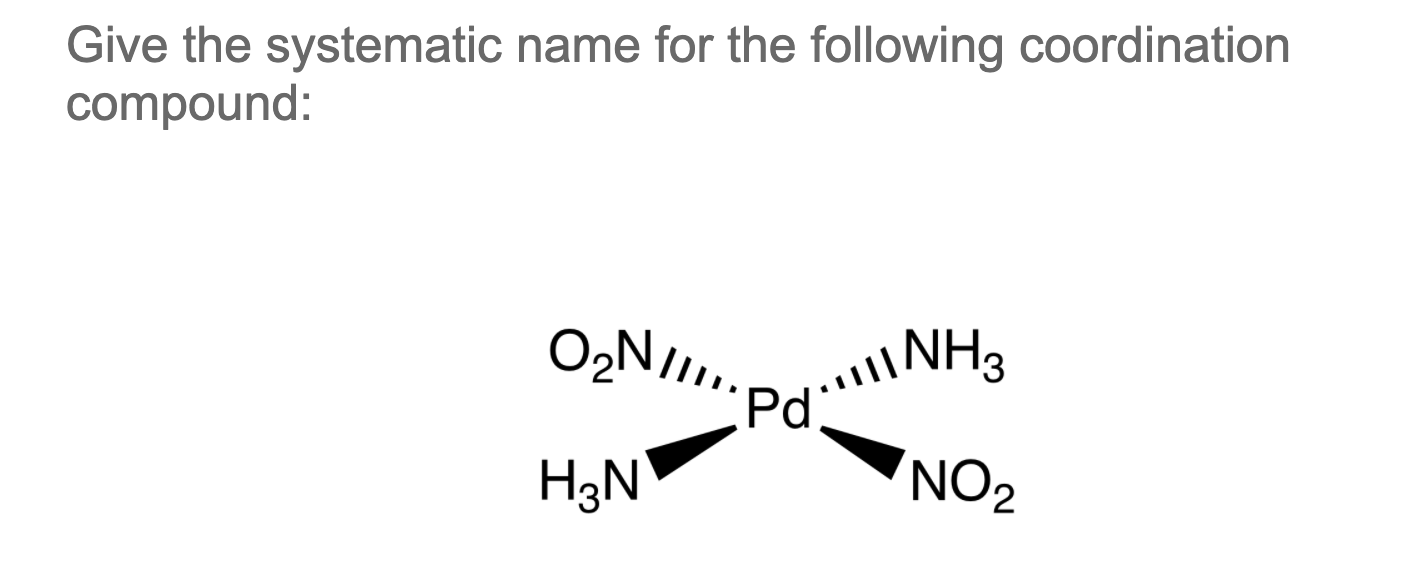
Solved Give the systematic name for the following
Notice that the mono-prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The \(\ce{S_2Cl_2}\) emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono-and the a of hepta-are dropped from the name when paired with oxide.